Chemical reactions involve bond cleavage, the breaking of chemical bonds between atoms. This fundamental process can occur via heterolytic cleavage, where electrons are unequally distributed, or homolytic cleavage, where electrons are shared equally. Bond cleavage often produces radicals or free radicals, highly reactive species with unpaired electrons. These radicals play a crucial role in chain reactions, where one reaction step leads to the initiation and propagation of subsequent reactions. The activation energy, the minimum energy required for a reaction to occur, is influenced by the bond strength and the type of bond cleavage involved. Understanding bond cleavage is essential for comprehending the mechanisms and energy requirements of chemical reactions.
Delving into the World of Chemical Reactions
Imagine a world where substances transform into entirely new substances. This magical process is known as a chemical reaction, the cornerstone of chemistry. Chemical reactions are like puzzles, where molecules rearrange like Lego blocks to create new compounds with distinct properties.
At the heart of every chemical reaction lies the concept of bond cleavage, the breaking apart of chemical bonds that hold atoms together. Think of bond cleavage as the separation of two dancers who were once gracefully entwined. This bond cleavage can happen in two different ways: heterolytic and homolytic.
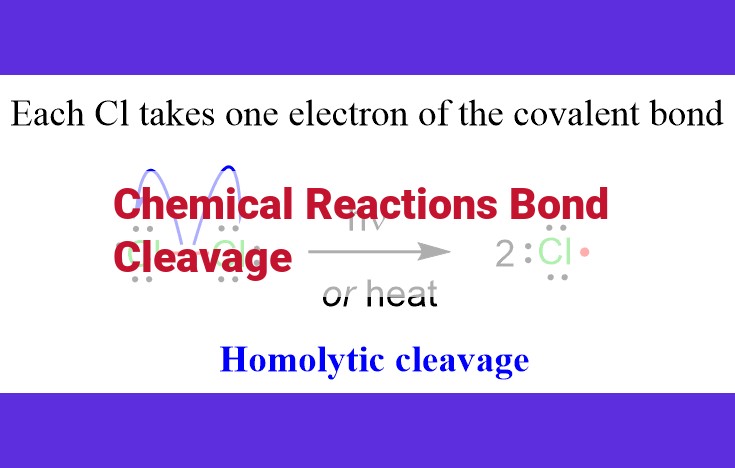
Heterolytic bond cleavage is like a graceful waltz, where one atom takes both electrons from the bond, becoming negatively charged. This leads to the formation of charged particles called ions. On the other hand, homolytic bond cleavage is more like a forceful break-up, with each atom taking one electron, creating two neutral but highly reactive particles known as free radicals.
Types of Bond Cleavage
- Heterolytic Bond Cleavage:
- Explain the mechanism of heterolytic bond cleavage.
- Discuss the formation of radicals in this process.
- Homolytic Bond Cleavage:
- Explain the mechanism of homolytic bond cleavage.
- Introduce free radicals as products of this cleavage.
Types of Bond Cleavage: Driving Chemical Reactions
The dance of atoms reorganizing themselves during chemical reactions relies on the delicate art of bond cleavage. Like a master chef breaking down ingredients to create a new dish, chemical reactions involve breaking and forming bonds between atoms. Two distinct types of bond cleavage pave the way for these transformations:
1. Heterolytic Bond Cleavage: The Unequal Breakup
Imagine a tug-of-war between two atoms sharing electrons in a covalent bond. In heterolytic bond cleavage, one atom emerges as the victor, claiming both electrons and leaving the other atom with a charge. This process resembles a divorce, where one partner walks away with the house and the other with a broken heart.
2. Homolytic Bond Cleavage: The Peaceful Separation
Unlike heterolytic bond cleavage, homolytic bond cleavage is a more amicable affair. Here, both atoms involved in the bond share the electrons equally, leading to the formation of uncharged species known as free radicals. These radicals, like untethered spirits, possess unpaired electrons that crave chemical companionship.
Radicals: The Lonely Hearts of Chemistry
Free radicals are chemical loners, perpetually seeking to complete their electron shells. Their instability makes them highly reactive, eager to form new bonds with any available electron-rich species.
Chain Reactions: A Domino Effect of Bond Cleavage
Some chemical reactions unfold like a chain reaction, where the breaking of one bond triggers a cascade of subsequent bond cleavages. These chain reactions feature three distinct stages:
- Initiation: A radical or other reactive species kicks off the chain reaction.
- Propagation: The radical reacts with another molecule, creating a new radical and continuing the chain.
- Termination: Two radicals collide and recombine, quenching the chain reaction.
Bond Cleavage and Activation Energy: The Key to Unleashing Reactions
Every chemical reaction requires a certain amount of energy to get started, known as the activation energy. Bond cleavage plays a crucial role in determining this energy barrier. Bonds that are weaker (require less energy to break) result in lower activation energies and faster reactions.
By understanding the types of bond cleavage, we unravel the secrets of chemical reactions. From the heterolytic breakup to the homolytic split, each cleavage mechanism introduces unique characteristics and sets the stage for the dance of atoms that orchestrates the wonders of chemistry.
Radicals and Free Radicals: The Key Players in Chemical Reactions
As we explore the captivating world of chemical reactions, we encounter two intriguing entities: radicals and free radicals. These enigmatic molecules hold immense significance in the realm of bond cleavage, playing pivotal roles in shaping the course of chemical transformations.
Defining Radicals and Free Radicals
Radicals and free radicals are molecular species that possess unpaired electrons. This unique characteristic endows them with a remarkable reactivity and the potential to participate in various chemical reactions. Radicals, often denoted with a single dot (e.g., Cl·), typically have one unpaired electron, while free radicals possess multiple unpaired electrons (e.g., Br2●-).
Role in Bond Cleavage Reactions
Radicals and free radicals act as catalysts for bond cleavage, initiating a domino effect of chemical transformations. In heterolytic bond cleavage, one of the electrons is transferred to a nucleophile, resulting in the formation of positively and negatively charged fragments. In contrast, homolytic bond cleavage occurs when each atom involved in the bond retains one of the electrons, leading to the formation of two free radicals.
Stability, Reactivity, and Properties of Free Radicals
Free radicals are inherently unstable due to their unpaired electrons, which makes them highly reactive. They exhibit a strong tendency to react with other molecules, seeking to stabilize their electronic configurations. The stability and reactivity of free radicals depend on various factors, including the number of unpaired electrons, the stability of the resulting radicals, and the surrounding environment.
Radicals and free radicals are indispensable players in the multifaceted world of chemical reactions. Their ability to participate in bond cleavage reactions underscores their importance in shaping the molecular landscape. Understanding these enigmatic entities provides a deeper appreciation for the intricate mechanisms that govern the chemical transformations that occur around us.
Chain Reactions: A Story of Radical Transformations
In the realm of chemistry, certain reactions unfold like captivating tales of transformation. These reactions, known as chain reactions, are characterized by a distinctive domino effect, where one reaction leads to another in a mesmerizing cascade.
Initiation: Sparking the Chain
Chain reactions begin with a crucial event called initiation. It’s like lighting the fuse of a firecracker, where an external energy source triggers the first bond cleavage. This initial cleavage creates free radicals, highly reactive species that possess an unpaired electron.
Propagation: The Unfolding Story
Once free radicals are unleashed, they embark on a mission to propagate the reaction. Like unstoppable messengers, they collide with other molecules, transferring their unpaired electron and triggering further bond cleavages. This propagation step repeats itself, leading to a chain of reactions that can grow exponentially.
Termination: The Grand Finale
As the chain reaction progresses, it eventually reaches its conclusion with a final stage known as termination. This occurs when free radicals combine with each other or with stable molecules, neutralizing their unpaired electrons and halting the chain.
A Tale of Energy and Bond Cleavage
Chain reactions are intricately connected to another fundamental concept in chemistry: activation energy. This is the initial hurdle that must be overcome for a reaction to occur. In chain reactions, bond cleavage is the energy-intensive step that provides the necessary activation.
By understanding the mechanics of chain reactions, we gain insights into a wide range of chemical processes. From the combustion of fuels to the polymerizations that form plastics, these reactions are ubiquitous in our world. So, the next time you witness a chemical reaction, remember the captivating story of chain reactions unfolding behind the scenes.
Bond Cleavage and Activation Energy: Understanding the Spark that Ignites Reactions
In the realm of chemistry, reactions reign supreme, orchestrating the transformation of one substance into another. At the heart of these transformations lies bond cleavage, a pivotal process that determines whether a reaction will take place and at what speed.
Just as every lock has a key, activation energy acts as a gatekeeper in chemical reactions. It represents the minimum amount of energy required to break the bonds holding atoms together. Think of it as the spark that ignites the reaction, allowing molecules to dance and rearrange themselves to form new substances.
The relationship between bond cleavage and activation energy is intricate. Stronger bonds require more energy to break, resulting in higher activation energy. For example, in a covalent bond, where electrons are shared between atoms, the stronger the bond, the higher the activation energy required for cleavage.
Conversely, weaker bonds yield to lower activation energy. Ionic bonds, which involve the transfer of electrons between atoms, are generally weaker, resulting in lower activation energy for cleavage.
Understanding the interplay between bond cleavage and activation energy is crucial for predicting reaction rates and controlling chemical processes. By manipulating the activation energy, chemists can orchestrate reactions to proceed at desirable speeds and achieve optimal outcomes.
In conclusion, bond cleavage and activation energy are inseparable players in the world of chemical reactions. The strength of the bonds being broken directly influences the amount of energy required to overcome the activation energy barrier, shaping the speed and efficiency of chemical transformations.