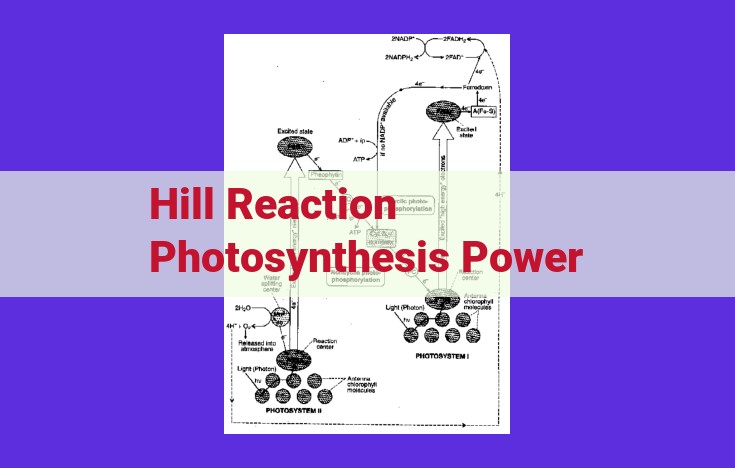
The Hill reaction, a pivotal process in photosynthesis, harnesses sunlight to generate a proton gradient across the thylakoid membrane. This gradient, a reservoir of energy, drives the synthesis of ATP through ATP synthase. The electron transport chain, a series of protein complexes, facilitates the flow of electrons from excited chlorophyll molecules, pumping protons across the membrane. These protons accumulate, creating a concentration gradient that fuels the ATP synthase’s activity, producing ATP—the universal energy currency of living cells.
Photophosphorylation: Harnessing Sunlight for Energy
- Introduction to the light-dependent reactions of photosynthesis
- Explain the electron transport chain (ETC) and proton pumping
Photophosphorylation: Harnessing Sunlight for Energy
Photosynthesis, the life-giving process that converts sunlight into energy, consists of two distinct stages: the light-dependent reactions and the Calvin cycle. It all begins with the light-dependent reactions, where sunlight is captured and transformed into chemical energy. This energy is then harnessed to produce ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate), essential molecules for the Calvin cycle that converts carbon dioxide into glucose.
Electron Transport Chain: The Conduit of Electron Flow
At the heart of the light-dependent reactions lies the electron transport chain (ETC), a series of protein complexes embedded in the thylakoid membrane of chloroplasts. These complexes act as electron carriers, passing electrons down the chain like a bucket brigade. As electrons flow through the ETC, their energy is used to pump protons (H+) from the stroma (fluid-filled space outside the thylakoid membrane) into the thylakoid lumen (space within the thylakoid membrane). This creates a proton gradient, a difference in proton concentration between the two compartments.
Proton Gradient: The Fuel for ATP Production
The proton gradient is the driving force behind ATP synthesis. ATP synthase, an enzyme complex located in the thylakoid membrane, harnesses this proton gradient. As protons flow back from the thylakoid lumen to the stroma through ATP synthase, their energy is used to synthesize ATP from ADP (adenosine diphosphate) and inorganic phosphate. This energy-rich ATP can then be utilized by the cell for various cellular processes.
Photophosphorylation, the process of converting sunlight into chemical energy through the electron transport chain and proton gradient, is a crucial step in photosynthesis. It provides the energy (ATP and NADPH) required for the Calvin cycle to produce glucose, the fundamental building block of life. This process underscores the remarkable ability of plants to harness sunlight and convert it into a form of energy that supports ecosystems worldwide.
Electron Transport Chain: The Conduit of Electron Flow
The electron transport chain (ETC) is a crucial component of photosynthesis, playing a central role in harnessing sunlight to generate energy. This sophisticated network of protein complexes acts as a conduit for electron flow, driving the creation of a proton gradient essential for producing ATP, the cell’s energy currency.
ETC Complexes and Their Functions:
- Complex I (NADH-Q oxidoreductase): Receives electrons from NADH, a high-energy electron carrier reduced during the light-dependent reactions of photosynthesis.
- Complex II (succinate-Q oxidoreductase): Alternative entry point for electrons from respiration, allowing for the integration of photosynthesis and cellular respiration.
- Complex III (cytochrome bc1 complex): Transfers electrons from ubiquinone (Q) to cytochrome c, releasing two protons into the intermembrane space.
- Complex IV (cytochrome c oxidase): Final electron acceptor, receiving electrons from cytochrome c and combining them with protons and oxygen to form water.
- ATPase activity: ATP synthase utilizes the proton gradient to drive the synthesis of ATP.
Proton Gradient and Membrane Potential:
As electrons pass through the ETC complexes, they release energy that pumps protons across the thylakoid membrane, creating a proton gradient. This gradient consists of a high concentration of protons in the intermembrane space and a low concentration within the thylakoid lumen. This creates an electrochemical gradient, with an electrical component (membrane potential) and a chemical component (pH gradient). The membrane potential provides the driving force for ATP synthesis.
Proton Gradient: The Fuel for ATP Production
In the bustling metropolis of cellular energy metabolism, proton gradients emerge as the driving force behind the production of ATP, the universal currency of cellular life. These gradients, like miniature hydroelectric dams, harness the flow of protons across biological membranes to generate the energy for ATP synthesis.
The Creation and Maintenance of Proton Gradients
Proton gradients arise from the asymmetric distribution of protons across a membrane. This asymmetry is meticulously engineered by an intricate interplay of proteins, including the electron-pumping complexes of the electron transport chain (ETC) and the proton-pumping activity of the ATPase enzyme.
The ETC, a molecular symphony, orchestrates the transfer of electrons through a series of protein complexes, creating a cascade of energy. This energy is harnessed to pump protons from the matrix of the cell into the intermembrane space, generating a proton concentration gradient.
The Role of ATPase Activity and Proton Influx
ATPase, a crucial gatekeeper of cellular energy, sits poised on the membrane. It harnesses the proton gradient to drive the synthesis of ATP from ADP and inorganic phosphate. As protons flow back down their concentration gradient into the matrix, they pass through ATPase, causing a conformational change that drives ATP synthesis.
This intricate dance of proton flow and ATPase activity sustains the proton gradient, ensuring a continuous supply of energy for cellular processes.
Harnessing the Proton Gradient for Cellular Energetics
The proton gradient is a universal energy currency in both photosynthesis and cellular respiration, the two major energy-generating pathways of life. In photosynthesis, the light-dependent reactions generate a proton gradient that drives the synthesis of ATP. In cellular respiration, the ETC pumps protons to generate a gradient that drives ATP synthesis in the mitochondria.
This elegant mechanism, chemiosmosis, underscores the fundamental role of proton gradients in cellular energetics. It is a testament to the ingenuity of nature, where the flow of protons through membranes powers the very essence of life.
ATP Synthase: Converting the Gradient into Energy
The final chapter in the captivating saga of chemiosmosis unfolds with the introduction of ATP synthase, the molecular maestro that orchestrates the conversion of a proton gradient into the energy currency of life: ATP.
Meet the F0-F1 Complex: A Masterful Machine
ATP synthase is a magnificent molecular machine composed of two fundamental components:
- F0: A hydrophobic membrane-spanning complex that forms a proton channel.
- F1: A hydrophilic headpiece that houses the catalytic site for ATP synthesis.
Proton Gradient: A Driving Force
The proton gradient established across the membrane by the electron transport chain serves as the driving force for ATP synthesis. As protons rush down their electrochemical gradient through the F0 channel, they encounter an ingenious mechanism:
Coupling Proton Flow to ATP Synthesis
Within the F1 headpiece, a rotor and stator configuration ensures that the flow of protons through the F0 channel is intricately coupled to the rotational movement of the F1 head. This rotation drives conformational changes in the catalytic site, facilitating the remarkable conversion of ADP + Pi into ATP.
Sequential Steps in ATP Synthesis
The process of ATP synthesis occurs in a series of sequential steps:
- Proton Binding: Protons bind to the rotor within the F0 complex.
- Rotor Rotation: The flow of protons induces the rotation of the rotor, causing conformational changes in the catalytic site of F1.
- ATP Formation: The conformational changes facilitate the binding of ADP and inorganic phosphate (Pi), allowing them to condense into ATP.
- ATP Release: The newly synthesized ATP molecules are released from the F1 headpiece, ready to fuel the energetic demands of the cell.
ATP: A Cellular Lifeline
ATP is the universal energy currency of cells, powering everything from muscle contraction to nerve conduction. ATP synthase plays an indispensable role in the generation of this cellular lifeline, ensuring that cells have the energy they need to thrive.
The Significance of Proton Gradients
Proton gradients are essential for both photosynthesis and cellular respiration, the two fundamental processes that generate energy for life. In photosynthesis, light energy is harnessed to pump protons across the thylakoid membrane, driving ATP synthesis through ATP synthase. In cellular respiration, the oxidation of glucose creates a proton gradient across the mitochondrial inner membrane, which is once again used to fuel ATP production by ATP synthase.
ATP synthase is a remarkable molecular machine that transforms the energy stored in a proton gradient into the energy-rich molecule ATP. This process is essential for life, providing cells with the energy they need to perform countless functions. The intricate choreography of proton flow, rotor rotation, and catalytic activity within ATP synthase underscores the elegance and complexity of the cellular world.
Chemiosmosis: The Energy Currency of Living Cells
Life on Earth thrives thanks to a remarkable process called chemiosmosis, which harnesses the power of proton gradients to drive essential cellular activities. From the verdant leaves of plants to the enigmatic depths of mitochondria, chemiosmosis plays a crucial role in cellular respiration and photosynthesis.
Proton Gradients: The Driving Force
Proton gradients are differences in the concentration of hydrogen ions (H+) across a membrane. These gradients act as a reservoir of chemical potential energy, similar to the water behind a dam. In chemiosmosis, the proton gradient is used to fuel the synthesis of ATP, the energy currency of cells.
Photosynthesis: Harnessing Sunlight’s Energy
Photosynthesis, the life-giving process that converts sunlight into chemical energy, relies heavily on chemiosmosis. In the light-dependent reactions, sunlight powers the electron transport chain (ETC), a series of protein complexes that creates a proton gradient across the thylakoid membrane. The resulting proton gradient drives the ATP synthase enzyme, which synthesizes ATP.
Cellular Respiration: The Powerhouse of the Cell
Cellular respiration is the process by which cells break down glucose to release energy. In the inner mitochondrial membrane, the ETC also plays a central role. As electrons flow through the ETC, they pump protons across the membrane, creating a proton gradient. Again, this gradient drives ATP synthase, generating ATP to power cellular functions.
The Significance of Proton Gradients
Proton gradients are essential not only for ATP synthesis but also for other cellular processes. For example, in certain bacteria, proton gradients drive the movement of nutrients into and out of the cell. The versatility of proton gradients highlights their importance in the intricate symphony of life.
Chemiosmosis is a fundamental process that underpins the energy metabolism of all living organisms. By harnessing the power of proton gradients, cells can efficiently convert chemical potential energy into ATP, the driving force behind countless cellular processes. From the photosynthetic leaves to the respiring cells, chemiosmosis powers the engines of life, ensuring the continuation of our vibrant planet.