Heat rejection science involves understanding the principles of heat transfer and its removal from systems or processes. It covers methods like convection, conduction, and radiation. Key concepts include heat sinks, thermal conductivity, and specific heat capacity. Practical applications include heat exchangers, cooling towers, and refrigeration cycles. Thermodynamics governs heat flow and rejection, with factors like temperature difference and material properties influencing the process. By comprehending heat rejection science, engineers design efficient thermal management systems, ensuring optimal operation and reliability in various industries.
Understanding the Significance of Heat Rejection
In the realm of engineering and industrial operations, heat rejection holds paramount importance. It refers to the process of dissipating excess heat from systems, components, and equipment to maintain their optimal performance and prevent damage.
The need for effective heat rejection is prevalent in diverse industries, including power generation, manufacturing, automotive, and electronics. In power plants, heat rejection is crucial for minimizing thermal losses and maximizing energy efficiency. In factories, it helps prevent overheating of machinery and ensures production continuity. In automobiles, efficient heat rejection safeguards engines and other components from damage caused by excessive temperatures. Electronic devices rely on heat rejection to prevent short-circuiting and ensure reliable operation.
Methods of Heat Rejection
Understanding the mechanisms of heat transfer is crucial for effectively rejecting heat from systems. Convection, conduction, and radiation are the three primary methods through which heat is transferred.
Convection involves the transfer of heat through the movement of a fluid, such as air or water. When a hot surface comes into contact with a cooler fluid, the fluid absorbs heat and rises, carrying the heat away from the surface. This process is commonly employed in many cooling applications, such as fans and heat sinks.
Conduction occurs when heat flows directly through a solid material. Heat transfer through conduction is driven by a temperature gradient, where heat flows from regions of higher temperature to regions of lower temperature. This process is utilized in heat sinks and other components designed to dissipate heat from electronic devices.
Radiation involves the emission of electromagnetic waves from a heated surface. Unlike convection and conduction, radiation does not require a medium to transfer heat. This makes it an effective method for heat rejection in vacuum environments or across large distances, such as in space applications.
By understanding these primary heat transfer mechanisms, engineers can design and implement effective heat rejection solutions for various applications. From cooling computer chips to managing heat in power plants, heat rejection plays a vital role in optimizing system performance and ensuring reliability.
**Important Concepts in Heat Rejection**
Understanding the fundamental concepts of heat rejection is paramount to effectively managing and dissipating heat in various industrial applications. Let’s explore some key terms that play a crucial role in this field.
Heat Sink: A device designed to absorb and dissipate heat from a heat-generating component. Heat sinks enhance heat transfer by increasing the surface area available for dissipation.
Thermal Conductivity: A material property that measures its ability to conduct heat. The higher the thermal conductivity, the more efficiently a material can transfer heat.
Thermal Resistance: The opposition to heat flow through a material or a system. Thermal resistance is inversely proportional to thermal conductivity.
Specific Heat Capacity: The amount of heat required to raise the temperature of a unit mass of a material by one degree. It indicates how much heat a material can absorb or release without a significant temperature change.
Latent Heat: The heat energy released or absorbed during a phase change (e.g., melting, freezing, vaporization, or condensation) without a change in temperature. Latent heat is important in processes like heat exchangers and evaporators.
By grasping these concepts, engineers and designers can optimize heat dissipation systems in various applications, ensuring efficient and reliable performance.
Applications of Heat Rejection: A Journey into Thermal Management
In the realm of engineering, the ability to efficiently reject heat plays a pivotal role in the design and operation of countless industrial systems and everyday devices. This process, known as heat rejection, is crucial for maintaining optimal performance, ensuring safety, and extending the lifespan of equipment.
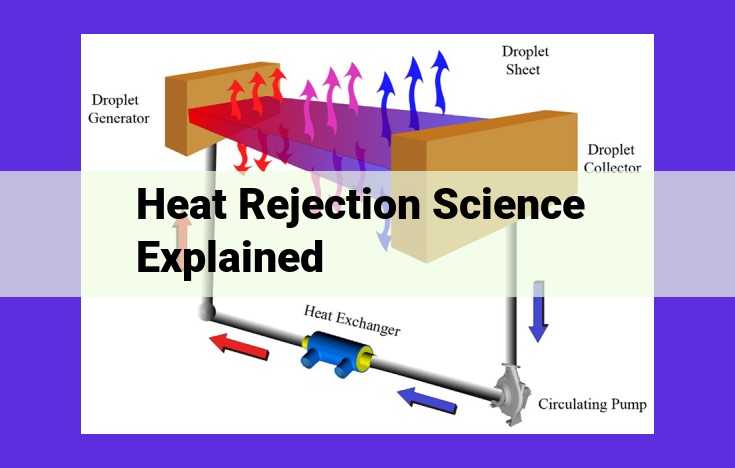
One of the most common applications of heat rejection is in the form of heat exchangers. These devices transfer heat between two fluids at different temperatures, allowing for cooling or heating. They find widespread use in power plants, refineries, food processing facilities, and HVAC systems to maintain precise temperature control.
Another essential application is the cooling tower. These tall, visible structures are commonly found in industrial complexes and large buildings. Cooling towers dissipate heat from water by evaporative cooling, which involves the release of heat as water vaporizes. This process effectively removes excess heat from industrial processes and air conditioning systems.
Condensers play a crucial role in refrigeration and air conditioning cycles. They convert refrigerant vapor into a liquid, releasing heat in the process. This heat is then typically rejected to the surrounding environment via air-cooled or water-cooled condensers.
Similarly, evaporators are essential components in refrigeration and heat pump systems. They absorb heat from the surrounding environment, converting liquid refrigerant into vapor. This vapor is then compressed and condensed, releasing heat again.
Finally, refrigeration cycles themselves are a prime example of heat rejection in action. Refrigerants circulate through a system, absorbing heat from the enclosed space and releasing it to the outside environment. This process effectively cools the space, creating a comfortable environment for homes, businesses, and other indoor spaces.
In all these applications, the science of heat rejection is paramount. Engineers carefully design and optimize systems to maximize heat transfer efficiency, ensuring that equipment operates within optimal temperature ranges and meets regulatory requirements.
Role of Thermodynamics in Heat Rejection
Understanding the Science Behind Heat Transfer
To delve into the fascinating world of heat rejection, it is essential to grasp the fundamental principles of thermodynamics. This branch of physics governs the flow of heat energy and plays a pivotal role in shaping the effectiveness of heat rejection systems.
Principles of Heat Flow
Thermodynamics dictates that heat flows from areas of higher temperature to areas of lower temperature. This principle, known as the Second Law of Thermodynamics, underpins the operation of all heat rejection systems. By manipulating temperature gradients, engineers can control and dissipate heat efficiently.
Factors Influencing Heat Transfer
The rate of heat transfer is influenced by several factors, including:
- Surface area: A larger surface area increases heat transfer due to the increased contact with the surrounding environment.
- Temperature difference: The greater the temperature difference between the heat source and the surroundings, the faster heat will transfer.
- Thermal conductivity: Materials with higher thermal conductivity, such as metals, facilitate faster heat transfer.
- Convection: Air currents and fluid flow can enhance heat transfer by carrying heat away from the source.
Implications for Heat Rejection
Thermodynamic principles drive the design and optimization of heat rejection systems. For instance, increasing the surface area of a heat sink maximizes heat dissipation. Similarly, using materials with high thermal conductivity facilitates the transfer of heat from the heat source to the cooling medium.
By understanding and leveraging the principles of thermodynamics, engineers can develop innovative technologies that effectively and efficiently reject heat, ensuring the optimal performance and reliability of various industries and applications.