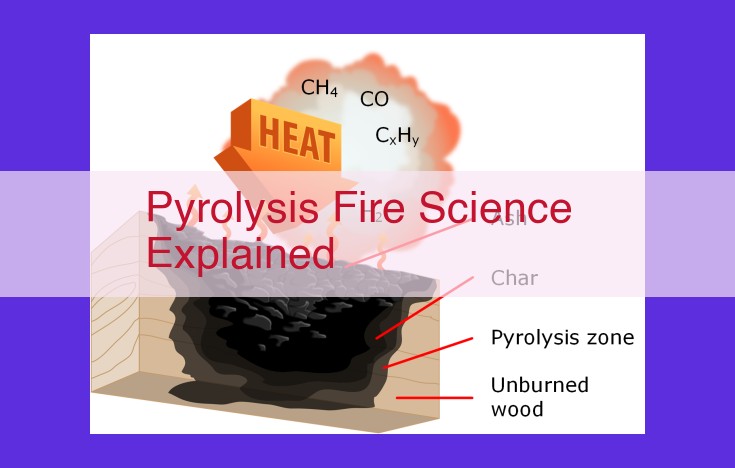
Pyrolysis, a key process in fire science, refers to the thermal decomposition of organic materials at elevated temperatures. During pyrolysis, the material undergoes primary decomposition, releasing gaseous products, followed by secondary decomposition of these volatiles. The products of pyrolysis include volatile gases and a solid residue called char, which vary depending on factors like heat of pyrolysis, activation energy, pyrolysis rate, and pyrolysis temperature. Understanding pyrolysis is crucial for predicting fire behavior, assessing fire risks, and developing fire safety measures.
Pyrolysis: Unraveling the Chemistry of Fire
In the realm of fire science, pyrolysis takes center stage as a crucial phenomenon that shapes the very nature of combustion. Simply put, pyrolysis is the process by which organic materials break down into simpler compounds under the influence of heat. This thermal decomposition plays a pivotal role in understanding fire behavior and designing effective fire safety strategies.
Pyrolysis is not merely a laboratory curiosity but a phenomenon that unfolds right before our eyes every time we light a candle, ignite a campfire, or witness the devastation caused by a raging inferno. By understanding the intricate chemistry behind pyrolysis, we gain invaluable insights into the behavior of fire and equip ourselves with the knowledge to prevent and mitigate its potentially catastrophic consequences.
Thermal decomposition process in detail.
Pyrolysis: A Thermal Decomposition Process
In the realm of fire science, pyrolysis takes center stage as a critical process that fuels flames and shapes fire behavior. It’s a fascinating thermal decomposition phenomenon where organic materials undergo a profound transformation under intense heat.
The Breakdown
As heat intensifies, the molecular bonds within organic compounds begin to weaken and break down. This intricate process, known as thermal decomposition, sees complex molecules fragment into simpler gases and vapors. The rate at which this decomposition occurs is influenced by several factors, including the heat of pyrolysis, the activation energy, and the temperature itself.
Energy and Activation
The heat of pyrolysis refers to the amount of energy required to initiate decomposition. Activation energy, on the other hand, represents the minimum energy threshold below which decomposition cannot occur. Once the temperature surpasses this threshold, the decomposition process gains momentum.
Rate and Temperature
The speed at which decomposition takes place is known as the pyrolysis rate. This rate is directly proportional to the temperature. As the temperature increases, decomposition accelerates, leading to a faster release of volatile gases and vapors.
The Sub-Process: An Overview
Pyrolysis can be further subdivided into two distinct stages: primary pyrolysis and secondary pyrolysis. Primary pyrolysis involves the initial decomposition of organic compounds into gaseous and vapor products. Secondary pyrolysis, on the other hand, refers to the subsequent decomposition of these volatile products into even simpler substances.
Related concepts: decomposition, heat of pyrolysis, activation energy, pyrolysis rate, and pyrolysis temperature.
Understanding the Fire’s Dance: A Journey into Pyrolysis
In the heart of every blazing fire lies a captivating dance of molecular transformation known as pyrolysis. This process unravels the secrets of how natural materials break down into their fundamental components when subjected to intense heat. Join us on a journey into the world of pyrolysis, where we’ll unravel its intricate steps and delve into the fascinating science behind it.
Pyrolysis: The Heat-Induced Dance of Decomposition
Pyrolysis isn’t just any decomposition process; it’s a thermal decomposition that requires the presence of heat to break down organic compounds. This high-energy dance involves the breaking of chemical bonds, leading to the creation of volatile gases and solid char.
Within the realm of pyrolysis, we encounter several key concepts:
- Decomposition: The fundamental process of breaking down complex structures into simpler ones.
- Heat of Pyrolysis: The energy required to initiate decomposition.
- Activation Energy: The minimum energy level that must be overcome for decomposition to occur.
- Pyrolysis Rate: The speed at which decomposition takes place.
- Pyrolysis Temperature: The temperature at which decomposition becomes significant.
Primary and Secondary: Pyrolysis in Two Acts
Pyrolysis unfolds in two distinct stages: primary and secondary. Primary pyrolysis holds center stage, where organic compounds undergo decomposition, releasing gaseous and vapor products known as volatiles. This initial act sets the stage for secondary pyrolysis, where the volatiles further decompose into smaller molecules.
Throughout both stages, concepts like decomposition, pyrolysis rate, and pyrolysis temperature play pivotal roles in shaping the decomposition process.
Products of the Dance: Volatiles and Char
The dance of pyrolysis culminates in the formation of two distinct products:
- Volatiles: The gaseous and vapor products released during primary pyrolysis, which include gases like carbon monoxide and hydrocarbons.
- Char: The solid residue left behind after pyrolysis, composed primarily of carbon.
Once again, decomposition, pyrolysis rate, and pyrolysis temperature influence the specific characteristics of each product.
The Influencers: Factors Shaping Pyrolysis
Just as in any dance, external factors play a crucial role in shaping the choreography of pyrolysis. Key factors that hold sway over this fiery transformation include:
- Heat of Pyrolysis: The energy required to ignite decomposition.
- Activation Energy: The hurdle that must be overcome for decomposition to begin.
- Pyrolysis Rate: The tempo of decomposition, dictating how quickly the process progresses.
- Pyrolysis Temperature: The temperature at which decomposition becomes a prominent force.
Understanding these factors provides a deeper appreciation for the intricate dance of pyrolysis, allowing us to unravel the secrets of fire’s behavior and harness its transformative power.
Two Stages of Pyrolysis: A Tale of Decomposition and Transformation
In the world of fire science, pyrolysis plays a pivotal role in understanding the behavior of burning materials. This fascinating process involves the thermal decomposition of organic compounds, unlocking the secrets of how fires evolve and spread. Primary and secondary pyrolysis are the two distinct stages that shape this intricate journey.
Primary Pyrolysis: The Genesis of Decomposition
As a fire ignites, organic materials begin to undergo primary pyrolysis. This stage marks the initial decomposition of complex compounds into simpler gaseous and vapor products (volatiles). Heat absorption during this process drives the breakdown of chemical bonds, transforming the material into an embryonic mix of combustible gases.
Secondary Pyrolysis: A Cascade of Further Decomposition
The volatile gases released during primary pyrolysis do not remain idle. Instead, they become the fuel for a subsequent stage known as secondary pyrolysis. In this process, the volatiles undergo further decomposition, releasing additional gases and vapors. This cascade of decomposition fuels the growth and intensity of the fire, perpetuating its spread.
Key Concepts in Pyrolysis
To fully grasp the nuances of pyrolysis, several key concepts warrant attention:
- Decomposition: The breakdown of complex compounds into simpler ones.
- Pyrolysis rate: The speed at which decomposition occurs.
- Pyrolysis temperature: The temperature at which decomposition takes place.
Pyrolysis: A Journey into the Heart of Fire
In the realm of fire science, pyrolysis reigns supreme as the crucial process that transforms solid materials into volatile gases and a solid residue called char. Understanding this phenomenon is essential for comprehending the behavior of fire and how it affects our world.
A Thermal Dance: Pyrolysis Unraveled
Pyrolysis is a thermal decomposition process that occurs when a material is subjected to high temperatures in the absence of oxygen. As the temperature rises, the bonds within the material’s molecules begin to break, releasing volatile gases and leaving behind a solid residue.
The Stages of Pyrolysis: A Two-Part Symphony
Pyrolysis unfolds in two distinct stages: primary and secondary. Primary pyrolysis involves the decomposition of the material’s organic compounds, releasing volatile gases and a solid residue. These gases can then undergo further decomposition in the secondary stage, known as secondary pyrolysis.
Products of Pyrolysis: Unveiling the Aftermath
The primary products of pyrolysis are volatile gases and vapors, which are released as the material decomposes. These gases include combustible species such as carbon monoxide and methane, as well as other toxic compounds. The solid residue left behind is char, which is composed primarily of carbon.
Factors that Shape Pyrolysis: A Balancing Act
Several key factors influence the pyrolysis process, including:
- Heat of pyrolysis: The energy required to break the molecular bonds and initiate decomposition.
- Activation energy: The minimum energy level required to start the pyrolysis reaction.
- Pyrolysis rate: The speed at which the material decomposes.
- Pyrolysis temperature: The temperature at which pyrolysis occurs and the extent of decomposition.
Pyrolysis is a complex phenomenon that plays a critical role in fire science. Understanding its processes, products, and influencing factors empowers us to harness its potential for various practical applications, including fire safety, waste management, and the production of renewable fuels. By embracing the power of pyrolysis, we unlock the secrets of fire and pave the way for a more sustainable future.
Gaseous and vapor products (volatiles) released from primary pyrolysis.
Understanding the Delights of Pyrolysis: Unraveling the Secrets of Fire Science
In the realm of fire science, pyrolysis reigns supreme as the gatekeeper of combustion. It’s the magical process that transforms everyday materials into volatile vapors, igniting the dance of flames that illuminate our nights and keep us warm.
Pyrolysis: A Fiery Transformation
Pyrolysis, the thermal decomposition of materials, is the furnace that breathes life into fire. As heat intensifies, the molecular bonds of organic compounds snap, giving birth to a symphony of vapors and gases. These volatiles, the essence of fire, ignite and spread, fueling the inferno.
The Two Faces of Pyrolysis: Primary and Secondary
Pyrolysis unfolds in two captivating stages: primary and secondary. During primary pyrolysis, organic matter surrenders to heat, releasing volatile vapors. These fleeting molecules are a heady blend of gases and vapors, a tantalizing cocktail that ignites with ease.
As the temperature escalates, the drama intensifies. In secondary pyrolysis, these volatile vapors encounter the inferno’s embrace, undergoing further decomposition. This fiery dance transforms them into an array of combustible gases, propelling the flames towards their peak.
A Symphony of Products: Volatiles and Char
Pyrolysis leaves behind a testament to its transformative power: the solid residue known as char. Char, a testament to the relentless heat, endures as a reminder of the once vibrant material.
Unveiling the Factors that Shape Pyrolysis
The delicate dance of pyrolysis is influenced by a myriad of factors, each playing a pivotal role in shaping the fiery tapestry. Heat of pyrolysis, the energy required to ignite the decomposition, sets the stage. Activation energy, the minimum energy needed for the molecular bonds to break, determines the readiness of the material to succumb to the flames.
Pyrolysis rate, the tempo at which the transformation unfolds, dictates the intensity of the inferno. Finally, pyrolysis temperature, the thermometer of the fiery process, governs the progress and extent of the decomposition.
Embracing the Transformative Power of Pyrolysis
In the crucible of pyrolysis, the mundane becomes extraordinary, the familiar yields to the unknown. It’s the alchemy of fire science, a mesmerizing journey into the secrets of combustion, where understanding unlocks the power to harness and control the dance of flames.
Understanding Pyrolysis: The Heart of Fire Science
Pyrolysis, the thermal decomposition of materials, lies at the very core of combustion and fire science. When materials are exposed to intense heat, they undergo a series of complex chemical transformations, leading to their breakdown into simpler compounds.
Primary Pyrolysis: Unveiling the Hidden Treasure
The first stage of pyrolysis, known as primary pyrolysis, marks the initial decomposition of organic compounds. As heat penetrates the material’s structure, bonds between molecules begin to break, releasing a treasure trove of gaseous and vapor products known as volatiles. This volatile cocktail consists of a myriad of compounds, including hydrocarbons, alcohols, and acids.
Secondary Pyrolysis: A Symphony of Further Decomposition
As primary pyrolysis proceeds, the volatiles produced undergo a second round of decomposition, known as secondary pyrolysis. This stage further breaks down the volatile compounds into smaller fragments, creating a complex tapestry of gases and vapors. The rate and temperature at which these reactions occur play a crucial role in determining the composition of the final products.
The Residual Legacy: Solid Residue (Char)
After the whirlwind of pyrolysis subsides, a solid residue remains, known as char. This charred remnant represents the remnants of the original material that have resisted complete decomposition. Char is primarily composed of carbon and other inorganic materials, such as ash and minerals. Its properties, such as porosity and surface area, can significantly influence the behavior of fire and its spread.
Pyrolysis in Fire Science: Unveiling the Secrets of Heat-Induced Decomposition
Pyrolysis, a crucial process in fire science, describes the thermal decomposition of organic materials into gaseous and solid products. Understanding pyrolysis is essential for comprehending fire behavior and developing effective firefighting strategies.
Understanding Pyrolysis: A Thermal Decomposition Process
Pyrolysis is a complex process that occurs when combustible materials are exposed to elevated temperatures. As heat penetrates the material, the molecular bonds break down, initiating decomposition. Key concepts associated with pyrolysis include activation energy (the minimum energy required), pyrolysis rate (the speed of decomposition), and pyrolysis temperature (the temperature at which decomposition occurs).
Primary and Secondary Pyrolysis: A Two-Stage Process
Pyrolysis typically occurs in two stages:
- Primary Pyrolysis: In this initial stage, organic compounds decompose into gaseous volatiles. The pyrolysis rate and temperature are critical factors in determining the composition and quantity of volatiles produced.
- Secondary Pyrolysis: The volatile products from primary pyrolysis further decompose into smaller molecules. The pyrolysis rate and temperature also influence the extent of secondary pyrolysis.
Products of Pyrolysis: Volatiles and Char
Pyrolysis produces a range of gaseous and vapor products (volatiles) and a solid residue known as char. Volatiles include carbon monoxide, carbon dioxide, and hydrocarbons, while char is the remaining non-volatile material.
Factors Affecting Pyrolysis
The pyrolysis process is influenced by several factors, including:
- Heat of Pyrolysis: The energy required to break molecular bonds and initiate decomposition.
- Activation Energy: The minimum energy level needed for molecules to react and decompose.
- Pyrolysis Rate: The speed at which pyrolysis occurs, which depends on factors like temperature and material properties.
- Pyrolysis Temperature: The temperature at which decomposition occurs, which can vary depending on the material and other factors.
Understanding the Key Factors that Influence Pyrolysis
In the realm of fire science, pyrolysis plays a crucial role in understanding the behavior of materials during combustion. This process of thermal decomposition profoundly affects the release of volatile gases and the formation of solid char, shaping the dynamics of fire spread and the generation of combustion products.
Heat of Pyrolysis: The Energy Required for Decomposition
The heat of pyrolysis represents the energy required to break down the chemical bonds holding a material together. This energy is supplied through external heat sources, such as flames or heat transfer from surroundings. The higher the heat of pyrolysis, the more energy is needed to initiate and sustain decomposition.
Activation Energy: The Minimum Energy Level for Decomposition
Activation energy is the minimum energy level that must be overcome for pyrolysis to occur. This threshold determines the rate at which decomposition proceeds. Materials with lower activation energies will pyrolyze more readily compared to those with higher activation energies.
Pyrolysis Rate: The Decomposition Speed
The pyrolysis rate measures the speed at which a material decomposes. This rate is influenced by multiple factors, including the heat of pyrolysis, activation energy, and material properties. Faster pyrolysis rates result in more rapid release of volatiles and char formation.
Pyrolysis Temperature: The Temperature at Which Decomposition Occurs
Pyrolysis temperature is the temperature at which decomposition becomes significant. This temperature varies depending on the material’s composition, heat of pyrolysis, and activation energy. Higher temperatures generally lead to faster pyrolysis rates and the release of more volatile products.
By comprehending these key factors that influence pyrolysis, fire scientists can better predict and control the combustion behavior of materials, mitigate fire hazards, and optimize combustion processes for various applications.
Understanding the Heat of Pyrolysis: The Fire’s Energy-Hungry Process
In the realm of fire science, pyrolysis holds a pivotal role, breaking down organic compounds into combustible gases and solid residue. Fueling this transformative process is the heat of pyrolysis, a critical factor that governs the rate and extent of decomposition.
就像开启一碗汤一样,热量是引发分解反应的火花。 热量能量作用于材料的分子键,使它们松动并最终断裂。这个过程需要一定量的能量,称为 热量。材料的不同决定了分解所需的热量值。例如,木材的热量比塑料低,这意味着它更容易燃烧。
热量不仅是启动分解反应的关键,它还影响着反应的 速度。更高的热量会加速分解过程,产生更多可燃物质。然而,如果热量太高,分解可能会失控,导致材料迅速燃烧。
Understanding Pyrolysis: The Key to Fire Science
In the realm of fire science, pyrolysis holds a pivotal position. It is a complex thermal decomposition process that plays a crucial role in combustion and fire spread. Delving into the depths of pyrolysis will illuminate its significance and provide a comprehensive understanding of fire behavior.
Pyrolysis: A Thermal Decomposition Process
Imagine a building engulfed in flames. As the inferno rages, materials within the structure undergo a remarkable transformation known as pyrolysis. This process involves the thermal decomposition of organic compounds, breaking them down into smaller molecules. Heat from the fire acts as a catalyst, triggering a cascade of reactions that release energy and produce volatile gases.
Activation Energy: The Barrier to Decomposition
To initiate pyrolysis, materials must overcome a certain energy threshold known as activation energy. Think of it as a lock that needs a key to open. Once this barrier is surpassed, the decomposition process can proceed. The activation energy required varies depending on the type of material and its chemical structure.
Primary and Secondary Pyrolysis
Pyrolysis unfolds in two distinct stages: primary and secondary. In primary pyrolysis, the organic compounds break down into volatile gases. These gases are highly flammable and contribute to the spread of fire. Secondary pyrolysis involves the further decomposition of these volatiles, producing smaller molecules and char – a solid residue that remains after pyrolysis.
Products of Pyrolysis: Volatiles and Char
The decomposition process yields a diverse array of products. Volatiles, primarily consisting of gases and vapors, escape from the material, fueling the fire. Char, on the other hand, remains as a solid residue, exhibiting reduced flammability and serving as an insulator.
Factors Affecting Pyrolysis
Numerous factors influence the pyrolysis process, including the heat of pyrolysis, activation energy, pyrolysis rate, and pyrolysis temperature. The heat of pyrolysis represents the energy required for decomposition, while activation energy determines the minimum energy level needed for the process to initiate. The pyrolysis rate measures the speed of decomposition, and the pyrolysis temperature indicates the temperature at which decomposition occurs. These parameters collectively shape the behavior of pyrolysis and its impact on fire dynamics.
Pyrolysis: The Decomposing Dance of Matter
In the realm of fire science, pyrolysis takes center stage, orchestrating the transformation of materials into their elemental components. A fascinating process, pyrolysis is the thermal decomposition of organic matter under the fiery embrace of heat, a dance of molecules and energy.
As heat intensifies, the molecular bonds within a substance begin to shake and sway. This frenzy of molecular motion weakens the bonds, leading to their eventual rupture. The original substance disintegrates into a myriad of smaller molecules, giving birth to a new chemical landscape.
The decomposition speed, known as the pyrolysis rate, is a crucial factor in this molecular ballet. It dictates how quickly the parent substance breaks down into its constituent parts. A higher pyrolysis rate translates to a faster decomposition process, while a lower rate indicates a more gradual breakdown.
Various elements influence the pyrolysis rate, each playing a unique role in the molecular choreography:
- Heat of pyrolysis: The energy required to initiate decomposition.
- Activation energy: The minimum energy level needed to trigger the molecular breakdown.
- Pyrolysis temperature: The specific temperature at which the decomposition process flourishes.
These factors orchestrate the rhythm of pyrolysis, influencing the speed at which matter relinquishes its original form and embarks on a new molecular journey.
Pyrolysis: Unraveling the Fire’s Chemical Dance
1. Pyrolysis: The Secret Ingredient in Fire
Pyrolysis, a fascinating process that holds the key to understanding fire, is the thermal decomposition of organic materials in the absence of oxygen. Imagine a symphony of chemical reactions where molecules break down under intense heat, releasing energy and creating a cascade of new substances.
2. Stages of Pyrolysis: A Tale of Two Acts
Pyrolysis unfolds in two distinct acts:
- Primary Pyrolysis: Organic compounds decompose, releasing gaseous and vapor products known as volatiles. This is the first chapter of the pyrolysis story.
- Secondary Pyrolysis: Volatiles dance in the flames, further decomposing into smaller molecules.
3. Products of Pyrolysis: A Symphony of Molecules
The products of pyrolysis are a diverse ensemble of substances:
- Volatiles: These are the gaseous and vapor products released during primary pyrolysis, carrying essential components for combustion.
- Char: This solid residue, the last remnant of the organic material, provides fuel for the fire’s glowing ember.
4. Factors Shaping the Pyrolysis Play
The pyrolysis process is influenced by a quartet of key factors:
- Heat of Pyrolysis: The energy required to break down the molecular bonds.
- Activation Energy: The threshold energy level that triggers decomposition.
- Pyrolysis Rate: The speed at which the decomposition occurs.
- Pyrolysis Temperature: The temperature at which the molecular dance of pyrolysis takes place. (This factor is not covered in the original outline, but it is an important aspect of pyrolysis.)